Abstract
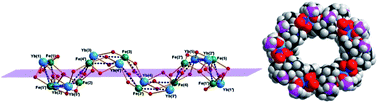
Five [FeIIImYbIIIn] Cyclic Coordination Clusters (CCCs) which show completely different arrangements of their cyclic cores have been synthesised and characterised. These represent five out of seven compounds which were obtained by fine-tuning the reaction conditions (FeIII-source, LnIII-salt, chiral/nonchiral aminoalcohol ligands) used previously to synthesise the non-cyclic [Fe2Yb2(OH)2(teaH)2(O2CPh)6] compound 1. Thus the five new CCCs [Fe5Yb3(μ3-OH)(teaH)7(O2CPh)8](CF3SO3)·10MeCN (m = 5; n = 2, 2), [Fe4Yb2(μ-OH)(μ-OMe)2(Me-teaH)4(O2CPh)7]·3CH3CN (m = 4; n = 2, 3), [Fe3Yb2(μ-OH)(Me-teaH)4(O2CPh)6]·MeCN (m = 3; n = 2, 4), [Fe4Yb2(Me-tea)4(Me-teaH)2(OTs)2]·MeCN·3MeOH (m = 4; n = 2, 5) and [Fe10Yb10(Me-tea)10(Me-teaH)10(NO3)10]·21MeCN (m = 10; n = 10, 6) as well as the binuclear complex [Yb2(tipH2)2(O2CPh)4] (7) are reported. The formation of compounds 2, 3 and 4, which were obtained using [Fe3O(O2CCPh)6(H2O)3]+ as the source of FeIII, can be rationalised in terms of cleaving the triangular {FeIII3O} starting material into mononuclear and dinuclear FeIII/benzoate species. Furthermore, it was found that for compounds 3–6, the interplay of ligand chirality, hydrogen-bonding interactions and the nature of anionic species present are decisive directional influences on the resulting nature of the cyclic compounds.

 Please wait while we load your content...
Please wait while we load your content...