Helical poly(arginine) mimics with superior cell-penetrating and molecular transporting properties†
Abstract
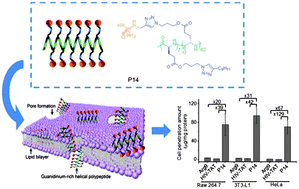
Poly(arginine) mimics bearing long hydrophobic side chains adopt a stable helical conformation and exhibit helix-related cell-penetrating properties. Elongating the polypeptide backbone length and increasing side chain hydrophobicity further increase the helicities of poly(arginine) mimics. These helical poly(arginine) mimics show superior cell membrane permeability up to two orders of magnitude higher than that of HIV-TAT peptide and excellent DNA and siRNA delivery efficiencies in various mammalian cells.

 Please wait while we load your content...
Please wait while we load your content...