Cation-directed enantioselective synthesis of quaternary-substituted indolenines†
Abstract
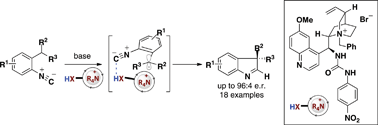
An asymmetric method for the synthesis of quaternary-substituted indolenines via a 5-endo-dig cyclization of an α-cyanocarbanion onto an isonitrile has been developed. This transformation relies on Brønsted acid

 Please wait while we load your content...
Please wait while we load your content...