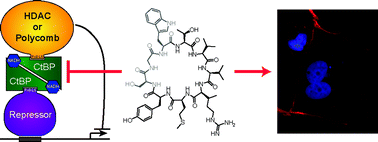
Identification of direct modulators of transcription factor protein–protein interactions is a key challenge for ligand discovery that promises to significantly advance current approaches to cancer therapy. Here, we report an inhibitor of NADH-dependent dimerization of the C-terminal binding protein (CtBP) transcriptional repressor, identified by screening genetically encoded cyclic peptide libraries of up to 64 million members. CtBP dimers form the core of transcription complexes associated with epigenetic regulation of multiple genes that control many characteristics of cancer cells, including proliferation, survival and migration. CtBP monomers also have distinct and critical cellular function, thus current experimental tools that deplete all forms of a targeted protein (e.g. siRNA) do not allow the cellular consequences of this metabolically regulated transcription factor to be deciphered. The most potent inhibitor from our screen (cyclo-SGWTVVRMY) is demonstrated to disrupt CtBP dimerization in vitro and in cells. This compound is used as a chemical tool to establish that the NADH-dependent dimerization of CtBPs regulates the maintenance of mitotic fidelity in cancer cells. Treatment of highly glycolytic breast cancer cell lines with the identified inhibitor significantly reduced their mitotic fidelity, proliferation and colony forming potential, whereas the compound does not affect mitotic fidelity of cells with lower glycolytic flux. This work not only links the altered metabolic state of transformed cells to a key determinant of the tumor cell phenotype, but the uncovered compound also serves as the starting point for the development of potential therapeutic agents that target tumors by disrupting the CtBP chromatin-modifying complex.
This article is Open Access
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...