Switching with orthogonal stimuli: electrochemical ring-closure and photochemical ring-opening of bis(thiazolyl)maleimides†
Abstract
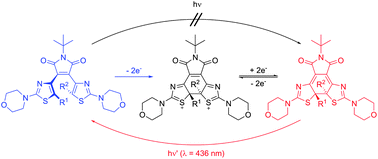
The photochemistry as well as electrochemistry of novel donor–acceptor bis(morpholinothiazolyl)–maleimides has been investigated. Proper substitution of these diarylethene-type molecular switches leads to the unique situation in which their ring-closure can only be accomplished electrochemically, while ring-opening can only be achieved photochemically. Hence, these switches operate with orthogonal stimuli, i.e. redox potential and light, respectively. The switch system could be optimized by introducing trifluoromethyl groups at the reactive carbon atoms in order to avoid by-product formation during oxidative ring closure. Both photochemical and electrochemical pathways were investigated for methylated, trifluoromethylated, and nonsymmetrical bis(morpholinothiazolyl)maleimides as well as the bis(morpholinothiazolyl)cyclopentene reference compound. With the aid of the nonsymmetrical “mixed” derivative, the mechanism of electrochemically driven ring closure could be elucidated and seems to proceed via a dicationic intermediate generated by two-fold

 Please wait while we load your content...
Please wait while we load your content...