An RNA catalyst that reacts with a mechanistic inhibitor of serine proteases†
Abstract
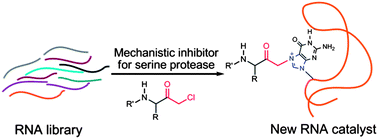
The enzymatic catalysis of difficult chemical reactions often requires the utilization of mechanisms completely different from those used in the uncatalyzed reaction. The catalytic triad of the serine proteases is an illustrative example for the cooperation of functional groups to achieve the hydrolysis of a very stable peptide bond via a covalent intermediate. Ribozymes for this demanding reaction that use similar mechanisms have neither been discovered nor developed to date. Here, we challenge a combinatorial RNA library with an active site-directed mechanistic inhibitor of serine proteases in order to identify RNA molecules with a chemical reactivity comparable to the residues in the catalytic center of thrombin. The adduct formed by this inhibitor is thought to mimic the first transition state in a complex reaction pathway and contains a weak electrophile. Indeed, various RNAs are enriched that accelerate their covalent attachment to the inhibitor, and several of them share a common motif that features a four-way junction. These RNAs specifically alkylate the N7-position of one particular guanosine, precisely recognizing structural features of the inhibitor. The selected RNAs may represent a valuable starting point for the further evolution of ribozymes with protease activity.

 Please wait while we load your content...
Please wait while we load your content...