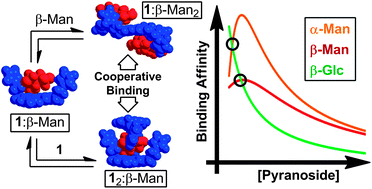
Tetrapodal receptor 1 relies upon structural flexibility to reveal new binding modes for saccharide recognition and to achieve unique pyranoside binding affinity and concentration dependent selectivity. The association constants, Kas, between 1 and eight pyranosides commonly found in cell surface glycans were measured in CDCl3 by 1H NMR titrations, revealing a preference for α- and β-octyl mannopyranosides (α-Man and β-Man). Whereas most of the pyranosides studied – α/β-octyl glucopyranoside (α/β-Glc), α/β-octyl galactopyranoside (α/β-Gal), and α/β-octyl N-acetylglucosaminopyranoside (α/β-GlcNAc) – bind 1 in a 1 : 1 stoichiometry at 25 °C, β-Man exclusively forms a 2 : 1 receptor–pyranoside complex. Alternatively, in an excess of pyranoside, 1 binds α- and β-Man in a 1 : 2 receptor : pyranoside stoichiometry with a high degree of positive cooperativity (K2/K1 ∼ 13.7 and 7.6 for α- and β-Man respectively) and selectivities as high as 16.8 : 1 α-Man : α-Gal. Moreover, this preference changes as a function of pyranoside concentration, favoring β-Glc at low concentration (<0.1 mM) and favoring mannosides at higher concentrations. The thermodynamic binding parameters (ΔH0 and ΔS0) reveal that the cooperativity in the second binding events drive the formation of 12:β-Man or 1:β-Man2 because of a decrease in unfavorable entropy upon each second binding event compared to the first. The structures of the complexes were determined by 1D and 2D 1H NMR spectroscopy in combination with molecular modeling. The 1:β-Man2 complex exhibits C2 symmetry, where both β-Man equivalents bind identical sites within 1, such that the pyranosides within the complex are symmetrically equivalent. Alternatively, 12:β-Man is a cage-like structure where only three of the aminopyrrolitic arms of the receptor are involved in binding, leaving a fourth available for further functionalization in later generation receptors. Multivalency and cooperativity are ubiquitous in Nature, and 1 utilizes these modes of recognition to achieve selectivity for monosaccharide residues.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...