Controlled tautomerism – switching caused by an “underground” anionic effect†
Abstract
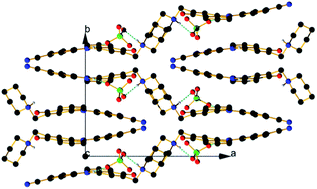
In a previous communication, we demonstrated a conceptual idea for a tautomeric switching system based on implementation of a flexible piperidine unit in 4-(phenyldiazenyl)naphthalen-1-ol (1). The results showed that a directed shift in the position of the tautomeric equilibrium can be achieved through protonation/deprotonation in a number of solvents. However, the effect of the counter ion in the process of protonation was never considered. The crystallographic analysis of protonated cyano and nitro derivatives of 4-(phenyldiazenyl)-2-(piperidin-1-ylmethyl)naphthalen-1-ol have shown an interesting and unexpected feature: the counter ion is captured in the process of protonation and the shift in the position of the tautomeric equilibrium is achieved through a bridged complex formation. To the best of our knowledge this is a rare example when controlled shift in the position of tautomeric equilibrium is achieved through anion complexation. The results from the solid state analyses are confirmed by NMR spectroscopy in solution and by quantum-chemical calculations.

 Please wait while we load your content...
Please wait while we load your content...