Bis(4′-(4-pyridyl)-2,2′:6′,2′′-terpyridine)ruthenium(ii) complexes and their N-alkylated derivatives in catalytic light-driven water oxidation
Abstract
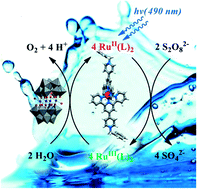
Hydrogen sulfate salts of [Ru(1)2]2+ where 1 = 4′-(4-pyridyl)-2,2′:6′,2′′-terpyridine and four N-alkylated derivatives [Ru(L)2]4+ were used as photosensitizers (λmax ∼510 nm) for water oxidation in light driven reactions with peroxydisulfate as a sacrificial electron acceptor and Na10[Co4(H2O)2(α-PW9O34)2] (Co4POM) as the catalyst in sodium borate buffers at pH 8.0 and 9.0. The N-substituents investigated were benzyl (L+ = 2+), ethyl (L+ = 3+), allyl (L+ = 4+) and 4-cyanobenzyl (L+ = 5+). The O2 yield in the presence of [Ru(L)2]4+ (L+ = 2+–4+) was comparable to that obtained in the presence [Ru(bpy)3]2+ (bpy = 2,2′-bipyridine) using light sources with λmax ≈ 490 nm. The ruthenium(III) complexes [Ru(1)2]3+ and [Ru(L)2]5+ (L+ = 2+–5+) are rather unstable in acidic conditions and could not be isolated. The most efficient photosensitizers [Ru(L)2]5+ (L+ = 2+ and 4+) were the least stable under weakly basic conditions (pH 9.0) with a half-life τ1/2 ∼ 10 ms. The stability of the complexes under photocatalytic turnover conditions is probably controlled by the rate at which ligand L+ is oxidized by Co4POM in its highest oxidation state.

 Please wait while we load your content...
Please wait while we load your content...