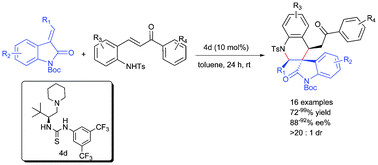
Organocatalyzed aza-Michael–Michael cascade reactions to construct spirooxindole tetrahydroquinolines with all-carbon chiral centers†
Abstract
An efficient organocatalytic aza-Michael–Michael cascade reaction for the asymmetric synthesis of highly functionalized spirooxindole tetrahydroquinolines has been reported through a formal [4+2] annulation strategy.

 Please wait while we load your content...
Please wait while we load your content...