Synthesis and evaluation of fluorescent cap analogues for mRNA labelling†
Abstract
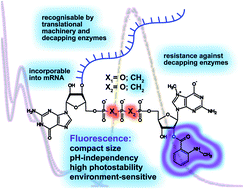
We describe the synthesis and properties of five dinucleotide fluorescent cap analogues labelled at the ribose of the 7-methylguanosine moiety with either anthraniloyl (Ant) or N-methylanthraniloyl (Mant), which have been designed for the preparation of fluorescent mRNAs via transcription in vitro. Two of the analogues bear a methylene modification in the triphosphate bridge, providing resistance against either the Dcp2 or DcpS decapping enzymes. All these compounds were prepared by ZnCl2-mediated coupling of a nucleotide P-imidazolide with a fluorescently labelled mononucleotide. To evaluate the utility of these compounds for studying interactions with cap-binding proteins and cap-related cellular processes, both biological and spectroscopic features of those compounds were determined. The results indicate acceptable quantum yields of fluorescence, pH independence, environmental sensitivity, and photostability. The cap analogues are incorporated by RNA polymerase into mRNA transcripts that are efficiently translated in vitro. Transcripts containing fluorescent caps but unmodified in the triphosphate chain are hydrolysed by Dcp2 whereas those containing a α-β methylene modification are resistant. Model studies exploiting sensitivity of Mant to changes of local environment demonstrated utility of the synthesized compounds for studying cap-related proteins.

 Please wait while we load your content...
Please wait while we load your content...