Triborate and pentaborate salts of non-metal cations derived from N-substituted piperazines: synthesis, structural (XRD) and thermal properties†
Abstract
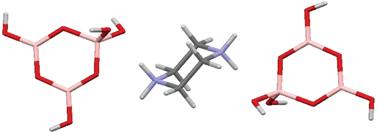
The synthesis and characterization of a triborate salt, [H2N(CH2CH2)2NH2][B3O3(OH)4]2 (1), and four pentaborate salts, [H2N(CH2CH2)2NH][B5O6(OH)4] (2a), [MeHN(CH2CH2)2NH][B5O6(OH)4] (2b), [MeHN(CH2CH2)2NMe][B5O6(OH)4] (2c) and [Me2N(CH2CH2)2NMe2][B5O6(OH)4]2 (2d) are described. TGA and DSC analysis (in air, 25–1000 °C) indicate that triborate 1 decomposes to B2O3via a multistage process, with the first stage (<250 °C) being dehydration to condensed polymeric hexaborate of approximate composition: [H2N(CH2CH2)2NH2][B6O10]. The pentaborates (2a–2d) are thermally decomposed to B2O3via a 2 stage process involving polymeric [NMC][B5O8]. The anhydrous polyborates were amorphous. BET analysis of materials derived from the thermolysis of 1 at 250, 400, 600, and 1000 °C, were all non-porous (surface area <1.8 m2 g−1). A single-crystal X-ray diffraction study of 1 showed that it contains isolated triborate(1−) anions in a structure comprised of alternating cationic and anionic layers held together via extensive H-bonds. Single-crystal XRD structural studies on pentaborate salts 2c and 2d are also reported.

 Please wait while we load your content...
Please wait while we load your content...