A general approach for the asymmetric synthesis of densely substituted piperidines and fully substituted piperidinones employing the asymmetric Mannich reaction as key step†
Abstract
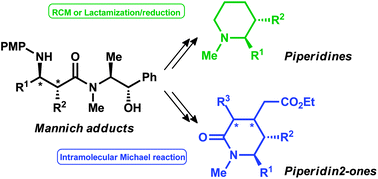
Two efficient and alternative protocols for the preparation of highly enantioenriched piperidine structures are reported. We have also developed a simple route to access to fully diastereo- and enantioenriched substituted piperidinones. The key step of all this synthesis relies on a diastereoselective Mannich reaction, employing the readily available aminoalcohol (+)-(S,S)-pseudoephedrine as a chiral auxiliary, which allows the preparation of β-aminocarbonyl compounds in high yield, diastereo and enantioselectivity and using easy-to-scale protocols.

 Please wait while we load your content...
Please wait while we load your content...