Understanding the unique interaction of amine-containing ionic compounds with SO2 for high absorption capacity†
Abstract
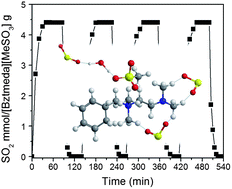
Control of the interaction between sorbents and acid gases (SO2 and CO2) is of prime importance for a fundamental understanding of acid–base chemistry to aid in the development of new absorbents. However, delicated and precise monitoring of the reaction dynamics by conventional one-dimensional (1D) spectroscopy is still challenging due to overlapping bands and restricted static information. Herein, we investigated the unique interactions of an amine-containing ionic compound, [Bztmeda][MeSO3], and SO2, which are related to the high absorption capacity and reversibility of the reaction, by principal component analysis (PCA) and two-dimensional infrared correlation spectroscopy (2D IR COS). The physical transition of [Bztmeda][MeSO3], arising from the hydrogen bonding interactions with SO2, could not be observed by 1D IR spectra, but was captured by PCA analysis. Synchronous 2D correlation spectra demonstrated that the absorption of SO2 was derived from the hydrogen bonding interactions between the tertiary amine groups of [Bztmeda] cations and the oxygen atoms of SO2 and/or between the sulfonate groups of [MeSO3] anions (bridged by water molecules) and the oxygen atoms of SO2. Asynchronous 2D correlation spectra decoupled the new bands of amine and sulfonate groups under the different chemical environments, showing the order of the actual sequence of the SO2 absorption processes.

 Please wait while we load your content...
Please wait while we load your content...