Thermal decomposition of Prussian Blue microcrystals and nanocrystals – iron(iii) oxide polymorphism control through reactant particle size
Abstract
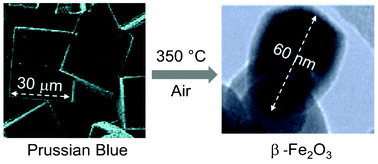
We present the first direct evidence that reactant particle size determines the mechanism of thermally induced solid-state reactions and can be used as the tuner of the product crystal structure. This control of the polymorphous character of the products through the particle size of precursor is demonstrated upon following the process of isothermal decomposition of Prussian Blue at 350 °C in air. The reaction mechanism was studied by 57Fe Mössbauer spectroscopy, X-ray powder diffraction, magnetization measurements, and transmission and scanning electron microscopy. The decomposition of Prussian Blue nanoparticles (10–15 nm) gave preferential formation of γ-Fe2O3 nanoparticles (10–20 nm), while Prussian Blue microcrystals (20–50 μm) afforded formation of β-Fe2O3 nanoparticles (50–70 nm). The final polymorphous composition of the iron(III) oxide product is thought to be driven by the different diffusion conditions for penetration of the reaction gas (oxygen) into the Prussian Blue crystals as well as release of the gas (dicyane) during the Prussian Blue decomposition. Therefore, the reactant particle size control provides a new method for the large-scale preparation of rare β-Fe2O3 nanoparticles.

 Please wait while we load your content...
Please wait while we load your content...