High U(vi) adsorption capacity by mesoporous Mg(OH)2 deriving from MgO hydrolysis†
Abstract
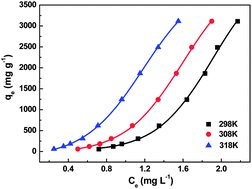
Hierarchical mesoporous–macroporous MgO (HMMM) is successfully synthesized by using a facile calcination method. Mesoporous Mg(OH)2 produced from HMMM hydrolysis in uranium solution was used to remove U(VI) from aqueous solutions. TEM confirms that mesoporous Mg(OH)2 partly retains interconnected porous structure of HMMM. Influence of U(VI) concentrations, adsorbent dosage, solution pH, salt concentration, contact time and temperatures on the adsorption properties were studied. The results indicate that mesoporous Mg(OH)2 exhibits excellent adsorption properties, particularly at high uranium concentration. The maximum adsorption capability of U(VI) is 3111 mg g−1 and the highest uranium removal efficiency is 99% at an initial uranium concentration of 500 mg L−1. We also demonstrate that HMMM hydrolysis process greatly improves adsorption capability of U(VI). The isotherm evaluations reveal that the Freundlich model attains a better fit to the experimental equilibrium data than the Langmuir model. The results of adsorption kinetics and adsorption mechanism for U(VI) indicate that chemical adsorption is the rate-limiting step. Furthermore, mesoporous Mg(OH)2 can be regenerated by using 1 M Na2CO3, which is reused with 9.3% loss of activity. Therefore, the mesoporous Mg(OH)2 is a potential absorbent in wastewater treatment because of its high uptake capability of U(VI).

 Please wait while we load your content...
Please wait while we load your content...