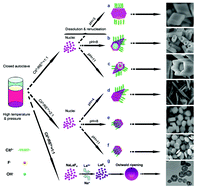
A novel and facile citrate-assisted hydrothermal approach has been developed for the shape-selective synthesis of fluoride microcrystals, including NaYF4 and YF3, by tuning the pH of the mother liquid. A series of controlled experiments demonstrate that three important factors, including the pH values of the mother solution and the amount of trisodium citrate in conjunction with the intrinsic character of rare-earth ions, are responsible for varied distinctive morphologies such as the truncated octahedrons, prismatic microtubes with unusual zigzag top ends, prismatic short rods with regular hexagonal concave centers, and thin plates. The possible formation mechanisms for products with various morphologies have been explored, and the mechanism based on selective adsorption and the cation exchange are proposed. The upconversion spectra of Yb/Er-doped fluoride samples with different shapes have been investigated. The shape effect on luminescence is attributed to the different crystal facet components of an anisotropic orientational microcrystal in which the crystal planes composed of the a-axis and c-axis have the difference in ion-space distance and crystallization quality.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...