In situ study of the impact of acidic and neutral deposition pH on alkane phosphate film formation and stability on TiO2†
Abstract
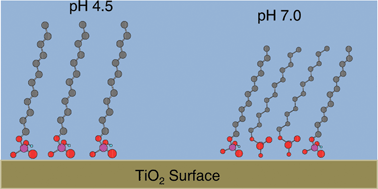
TiO2 has been used as a model system for the surface of medical implant devices based upon titanium alloys. Self Assembled Monolayers (SAM) can be applied via a specific

 Please wait while we load your content...
Please wait while we load your content...