Synthesis of linear and hyperbranched polyesters in Brønsted acid ionic liquids
Abstract
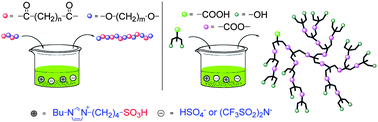
Imidazolium based Brønsted acid ionic liquids (BAILs) were proved to be very efficient solvents and catalysts for linear and hyperbranched polyester synthesis. Linear polyesters of diols and diacids with mass-average molar mass (Mw) up to 36 000 g mol−1 were obtained by the post-polycondensation of low molar-mass oligoesters at 110 °C in 15–30 min. Hyperbranched polyesters of 2,2-bis(hydroxymethyl)propanoic acid with a Mw of ca. 10 000 g mol−1 were yielded in 3-butyl-1-(butyl-4′-sulfonic acid)imidazolium hydrogen sulfate ([BBSIm]HSO4) in no more than 2 h, which is considerably shorter than the conventional polyesterification in the bulk. The degree of branching of the resulting hyperbranched polyesters increased with time. [BBSIm]HSO4 was found to be more suitable for polyesterification, as only the expected polyester was produced, while when polycondensations were carried out in the BAIL with Tf2N− anion ([BBSIm]Tf2N), side reactions were observed: ether formation and polymer scissions in linear polyester synthesis, and gelation in hyperbranched polyesterification.

 Please wait while we load your content...
Please wait while we load your content...