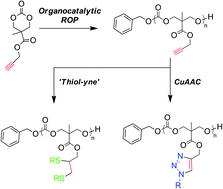
The synthesis of well-defined propargyl-functional poly(carbonate)s was achieved via the organocatalytic ring-opening polymerization of 5-methyl-5-propargyloxycarbonyl-1,3-dioxan-2-one (MPC) using the dual catalyst system of 1-(3,5-bis(trifluoromethyl)phenyl)-3-cyclohexylthiourea (TU) and 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU). The resulting homopolymers showed low dispersities and high end-group fidelity, with the versatility of the system being demonstrated by the synthesis of telechelic copolymers and block copolymers. The synthesized homopolymers with varying degree of polymerization were functionalized with a range of azides via copper-catalyzed Huisgen-1,3-dipolar addition or thiols via radical thiylation, to produce functional aliphatic poly(carbonate)s from a single polymeric scaffold.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?

 Please wait while we load your content...
Please wait while we load your content...