Polymer electrolyte membranes based on poly(arylene ether sulfone) with pendant perfluorosulfonic acid
Abstract
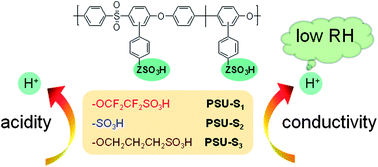
Poly(arylene
* Corresponding authors
a
Department of Chemistry and Chemical Biology, New York State Center for Polymer Synthesis, Rensselaer Polytechnic Institute, 110 8th Street, Troy, New York 12180, USA, 4505 Maryland Parkway, MC 454003, Las Vegas, Nevada 89154-4003, USA
E-mail:
baec@rpi.edu
Fax: +1 518-276-4887
Tel: +1 518-276-3783
b
School of Material Science and Engineering, Georgia Institute of Technology, 771 Ferst Dr, Atlanta, Georgia 30332-0245, USA
E-mail:
seungsoon.jang@mse.gatech.edu
Fax: +1 404-894-9140
Tel: +1 404-385-3356
c
Department of Materials Science and Engineering, The Pennsylvania State University, University Park, PA 16802, USA
E-mail:
Hickner@matse.psu.edu
Fax: +1 814-865-2917
Tel: +1 814-867-1847
d MST-7: Polymers and Coating Group, Los Alamos National Laboratory, Los Alamos, New Mexico 87544, USA
e MPA-11: Materials Physics and Applications, Sensors and Electrochemical Devices Group, Los Alamos National Laboratory, Los Alamos, New Mexico 87544, USA
Poly(arylene

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
Y. Chang, G. F. Brunello, J. Fuller, M. L. Disabb-Miller, M. E. Hawley, Y. S. Kim, M. A. Hickner, S. S. Jang and C. Bae, Polym. Chem., 2013, 4, 272 DOI: 10.1039/C2PY20666H
To request permission to reproduce material from this article, please go to the Copyright Clearance Center request page.
If you are an author contributing to an RSC publication, you do not need to request permission provided correct acknowledgement is given.
If you are the author of this article, you do not need to request permission to reproduce figures and diagrams provided correct acknowledgement is given. If you want to reproduce the whole article in a third-party publication (excluding your thesis/dissertation for which permission is not required) please go to the Copyright Clearance Center request page.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
