Substrate selectivity of high-activity mutants of human butyrylcholinesterase†
Abstract
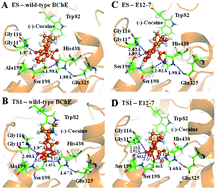
Cocaine is one of the most addictive drugs, and there is still no FDA (Food and Drug Administration)-approved medication specific for cocaine abuse. A promising therapeutic strategy is to accelerate cocaine metabolism, producing biologically inactive metabolites via a route similar to the primary cocaine-metabolizing pathway, i.e. cocaine hydrolysis catalyzed by butyrylcholinesterase (BChE) in plasma. However, the native BChE has a low catalytic efficiency against the abused cocaine, i.e. (−)-cocaine. Our recently designed and discovered A199S/F227A/S287G/A328W/Y332G mutant and other mutants of human BChE have a considerably improved catalytic efficiency against (−)-cocaine. In the present study, we carried out both computational modeling and experimental kinetic analysis on the catalytic activities of these promising new BChE mutants against other known substrates, including neurotransmitter acetylcholine (ACh), acetylthiocholine (ATC), butyrylthiocholine (BTC), and (+)-cocaine, in comparison with the corresponding catalytic activity against (−)-cocaine. Both the computational modeling and kinetic analysis have consistently revealed that all the examined amino acid mutations only considerably improve the catalytic efficiency of human BChE against (−)-cocaine, without significantly improving the catalytic efficiency of the enzyme against any of the other substrates examined. In particular, all the examined BChE mutants have a slightly lower catalytic efficiency against neurotransmitter ACh compared to the wild-type BChE. This observation gives us confidence in developing an anti-cocaine enzyme therapy by using one of these BChE mutants, particularly the A199S/F227A/S287G/A328W/Y332G mutant.

 Please wait while we load your content...
Please wait while we load your content...