Designing new symmetrical facial oligothiophene amphiphiles†
Abstract
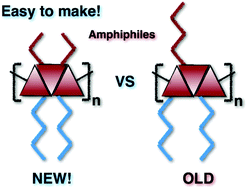
In this study we designed a new class of symmetrical facial oligothiophene amphiphiles, which could be obtained in fewer steps than for previously reported analogues, but still possess the specific substituent sequence to control their backbone curvature. This novel design allows the late-stage introduction of hydrophilic groups, aiding both purification and ease of structure variation. Following the new synthetic scheme, symmetrical ter- and sexi-thiophenes were synthesized, analysed and their properties were compared to their non-symmetrical analogues. Surprisingly, the self-assembly behaviour in water, aggregate morphologies and photo-physical properties turned out to be significantly different despite the same ratio of hydrophilic and hydrophobic substituents. The new substitution pattern resulted in a drastic decrease of the critical aggregation concentration and an increase of the aggregate size. The symmetrical positioning of the substituents also heavily influenced the photo-physical properties. The changes were observed as large blue shifts in the absorption and emission spectra in water when compared to similar regio-regular oligothiophene amphiphiles.

 Please wait while we load your content...
Please wait while we load your content...