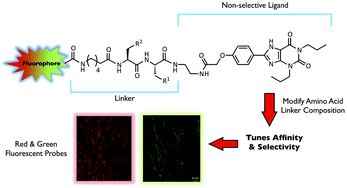
Conversion of a non-selective adenosine receptor antagonist into A3-selective high affinity fluorescent probes using peptide-based linkers†
Abstract
Advances in fluorescence-based imaging technologies have helped propel the study of real-time biological readouts and analysis across many different areas. In particular the use of fluorescent

 Please wait while we load your content...
Please wait while we load your content...