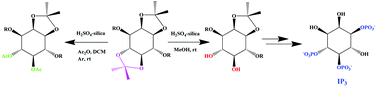
The involvement of natural phosphoinositols in various cellular signalling processes and the use of synthetic inositol derivatives in catalysis, supramolecular chemistry, natural product synthesis etc. gave momentum to myo-inositol chemistry. The presence of six secondary hydroxyl groups necessitates efficient protection–deprotection strategies for the synthesis of inositol derivatives. An important strategy for the initial protection of myo-inositol is the di-ketalization, which gives a mixture of three diketals, each having both cis-fused and trans-fused ketals. It is important to have methodologies either to selectively hydrolyze one of the two ketals or to convert one of the two acid labile ketals to an orthogonal base labile protecting group. By exploiting the difference in strain between trans-ketals and cis-ketals, we developed two operationally simple, high yielding methodologies for the chemoselective hydrolysis/acetolysis of trans-ketals (both isopropylidene and cyclohexylidene) of inositols, leaving the cis-ketal undisturbed, using cheap and easily preparable H2SO4-silica as the catalyst. Also, terminal ketal moieties of carbohydrates and acyclic polyols could be selectively hydrolyzed/acetolyzed leaving the internal ketals intact. The use of methanol as the solvent leads to chemoselective alcoholysis but the use of DCM and acetic anhydride leads to chemoselective acetolysis. Applying this methodology, a short synthesis of D-myo-inositol-1,4,5-trisphosphate has been achieved.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...