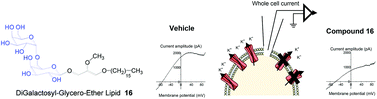
The recent discoveries of the involvement of SK3 channel in some cell motility mechanisms occurring in cancer disease have opened up the way to the synthesis of inhibitors that could reduce metastasis formation. On the basis of our recent previous works showing that both lactose-glycero-ether lipid (Ohmline) and some phosphate analogues (GPGEL) were efficient compounds to modulate SK3 channel activity, the present study, which found its inspiration in the structure of the natural glycolipid DiGalactosylDiacylGlycerol (DGDG), reports the incorporation of a digalactosyl moiety (α-galactopyranosyl-(1→6)-β-galactopyranosyl-) as the polar head of a glycero ether lipid. For the construction of the digalactosyl fragment, two synthetic approaches were compared. The standard strategy which is based on the use of the benzyl protecting group to produce 1→6 disaccharide unit, was compared with a second method that made use of the trimethylsilyl moiety as a protecting group. This second strategy, which is applied for the first time to the synthesis of (1→6)-disaccharide unit, presents a net advantage in terms of efficacy (better global yield) and cost. Finally, compound 16, which is characterized by a (1→6) DiGalactosyl unit (DG) as the polar head of the amphiphilic structure, was tested as a modulator of the SK3 channel activity. Patch-clamp experiments have shown that compound 16 reduced SK3 currents (−28.2 ± 2.0% at 5 μM) and cell migration assays performed at 300 nM have shown a reduction of cell migration (SK3 + HEK293T) by 19.6 ± 2.7%.
You have access to this article
 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?


 Please wait while we load your content...
Please wait while we load your content...