The effect of the 4-amino functionality on the photophysical and DNA binding properties of alkyl-pyridinium derived 1,8-naphthalimides†
Abstract
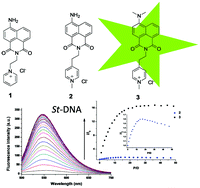
The synthesis and characterisation of two cationic pyridinium based 4-amino-1,8-naphthalimide derivatives (2 and 3) are described and compared to those of compound 1. The photophysical properties of 2 and 3 are shown to vary greatly with the solvent polarity and H-bonding ability. The dimethylamino substitution in 3 results in a weak quantum yield of fluorescence emission due to faster non-radiative deactivation of the excited singlet state than that seen for 2. As with 1, the fluorescence of 2 was found to be enhanced in its 1 : 1 complex with 5′-adenosine-monophosphate (5′-AMP) while it was partially quenched in its complex with 5′-guanosine-monophosphate (5′-GMP). In contrast, the fluorescence of 3 was enhanced (‘switched on’) in the presence of both adenine and guanine rich sequences. Linear and circular dichroism studies showed that each of 1, 2 and 3 binds to double-stranded DNA by intercalation. However, 2 and 3 do not show the preference for AT-rich DNA observed for 1. Comparative fluorescence studies with double stranded DNA show that the emission of 3 was 16 times enhanced in its DNA bound form, suggesting potential use of this structure as a spectroscopic probe for studying nucleic acid structure.

 Please wait while we load your content...
Please wait while we load your content...