Redox control of molecular motions in bipyridinium appended calixarenes†
Abstract
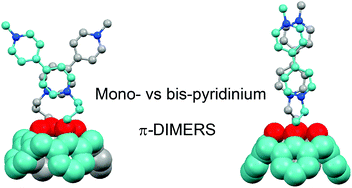
A series of redox-responsive architectures featuring two bipyridinium units introduced onto the lower rim of a calixarene skeleton has been synthesized. The redox-triggered intramolecular dimerization of the reduced bipyridiniums has been investigated by electrochemistry, spectroelectrochemistry and by theoretical chemistry. The spectroscopic signature of the intramolecular dimers was found to evolve significantly with the size of the

 Please wait while we load your content...
Please wait while we load your content...