Mechanisms of DNA damage by photoexcited 9-methyl-β-carbolines
Abstract
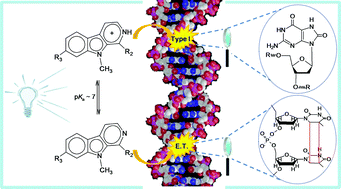
It has been well documented that β-carboline alkaloids, particularly the 9-methyl derivatives, are efficient photosensitizers. However, structure–activity relationships are missing and the photochemical mechanisms involved in the DNA photodamage still remain unknown. In the present work, we examined the capability of three 9-methyl-β-carbolines (9-methyl-norharmane, 9-methyl-harmane and 9-methyl-harmine) to induce DNA damage upon UVA excitation at physiological pH. The type and extent of the damage was analyzed together with the photophysical and binding properties of the β-carboline derivatives investigated. The results indicate that even at neutral pH most of the DNA damage is generated from the protonated form of the excited β-carbolines in a type-I reaction. Oxidized purine residues are produced in high excess over oxidized pyrimidines, single-strand breaks and sites of base loss. In addition, the excited neutral form of the β-carbolines is responsible for significant generation of cyclobutane pyrimidine dimers (CPDs) by triplet–triplet-energy transfer. In the case of 9-methyl-norharmane, the yield of CPDs is increased in D2O, probably due to less rapid protonation in the deuterated solvent.

 Please wait while we load your content...
Please wait while we load your content...