Synthetic use of the primary kinetic isotope effect in hydrogen atom transfer 2: generation of captodatively stabilised radicals
Abstract
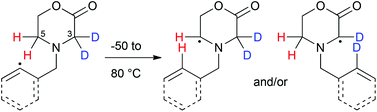
Using C-3 di-deuterated morpholin-2-ones bearing N-2-iodobenzyl and N-3-bromobut-3-enyl radical generating groups, only products derived from the more stabilised C-3, rather than the less stabilised C-5 translocated radicals, were formed after intramolecular 1,5-hydrogen atom transfer, suggesting that any kinetic isotope effect present was not sufficient to offset captodative stabilisation.

 Please wait while we load your content...
Please wait while we load your content...