Combinatorial tuning of peptidic drug candidates: high-affinity matriptase inhibitors through incremental structure-guided optimization†
Abstract
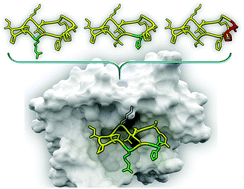
Herein we report a convenient strategy for the development of novel, highly-potent peptidic inhibitors of the trypsin-like serine protease matriptase based on the monocyclic variant of the sunflower trypsin inihibitor-1 (SFTI-1[1,14]). We screened SFTI-1[1,14] variants possessing incremental modifications of the parent peptide for beneficial binding properties. This compound library comprising 6 peptides and 16 triazole-containing peptidomimetics was established via structure-guided rational design and synthesized using a divergent strategy employing “copper-click” chemistry. The most favorable amino acid substitutions were combined in one framework yielding potent SFTI-1-derived matriptase inhibitor-1 (SDMI-1) and the truncated dodecapeptide variant (SDMI-2) with single-digit nanomolar inhibition constants. In silico studies indicated that the improved matriptase affinity compared to the parent peptide is caused by the successful establishment of additional favorable proton donor–acceptor interactions between basic inhibitor side chains and acidic residues on the surface of the target enzyme. SDMI-1 and 2 are potent inhibitors of the pharmaceutically relevant protease matriptase at a near physiological pH and, thus, may find applications in therapy or diagnostics.

 Please wait while we load your content...
Please wait while we load your content...