Electron densities of bexarotene and disila-bexarotene from invariom application: a comparative study†‡
Abstract
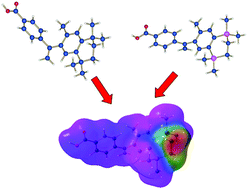
By the application of the invariom formalism, which provides aspherical atomic scattering factors, the electron densities of the RXR-selective

 Please wait while we load your content...
Please wait while we load your content...