The size of the aryl linker between two polyaza-cyclophane moieties controls the binding selectivity to ds-RNA vs. ds-DNA†
Abstract
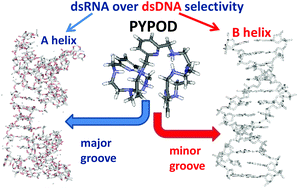
Aryl-linked (pyridine- vs. phenanthroline-) bis-polyaza pyridinophane scorpiands PYPOD and PHENPOD strongly bind to the double stranded DNA and RNA, whereby very intriguing RNA over DNA selectivity is finely tuned by aryl-linker length and aromatic surface. Moreover, PYPOD and PHENPOD dimer formation at high compound/polynucleotide ratios is highly sensitive to the fine interplay between the steric and binding properties of compound-dimers and the DNA minor groove/RNA major groove. That is demonstrated by significantly different induced CD spectra, which allow spectroscopic differentiation between various DNA/RNA secondary structures. A significantly higher (micromolar) antiproliferative effect of PYPOD and PHENPOD on human cell lines with respect to previously reported pyridine-based tripodal aliphatic polyamines is attributed to masked positive charges and increased hydrophobicity of novel compounds, resulting in more efficient membrane permeation and cellular uptake.

 Please wait while we load your content...
Please wait while we load your content...