Efficient synthesis of silylated 2,2-difluorostyrene derivatives through Suzuki–Miyaura cross-coupling of 2,2-difluoro-1-iodo-1-silylethenes†
Abstract
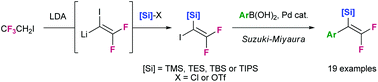
We report a new synthetic sequence for the preparation of silylated 2,2-difluorostyrene derivatives. This new route has numerous advantages over the previous one including enhanced scope, higher yields, ease of

 Please wait while we load your content...
Please wait while we load your content...