A computational study of the glycylserine hydrolysis at physiological pH: a zwitterionic versus anionic mechanism†
Abstract
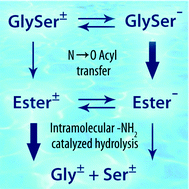
The hydrolysis of GlySer at physiological pH was investigated by modeling the most feasible reaction mechanisms in aqueous phase at the MP2/6-311+(2df,2p)//SMD-M06/6-311+(2df,2p) level of the theory. To refine the energies of the most relevant transition states along the reaction paths the cluster-continuum concept was adopted. The hydrolytic process could proceed through two competitive mechanisms involving either the zwitterionic or the anionic form of GlySer. The calculations suggest that at physiological pH the actual mechanism is most probably mixed, anionic–zwitterionic. In this reaction scheme the first stage of N→O acyl transfer involves the anionic form whereas the second stage, during which the resultant ester is hydrolyzed, most likely involves the zwitterionic ester form of GlySer. The energy requirement for the first reaction stage is estimated to be slightly lower than for the second one. The calculated activation parameters (e.g. ΔG# = 27.8 kcal mol−1) for the nucleophilic addition of a water molecule to the ester carbonyl group of the zwitterionic ester are in good agreement with the experimentally determined values at pD 7.4 (ΔG# = 28.7 kcal mol−1).

 Please wait while we load your content...
Please wait while we load your content...