Gold-catalyzed annulations of allenes with N-hydroxyanilines to form indole derivatives with benzaldehyde as a promoter†
Abstract
Gold-catalyzed syntheses of 2,3-disubstituted indole derivatives from N-hydroxyanilines and allenes are described; these reactions require benzaldehyde as an additive to generate nitrones in situ. Our control experiments indicate that nitrones and water were indispensable in the reactions whereas N-hydroxyanilines alone were inactive nucleophiles. This synthetic method is compatible with allenes and N-hydroxyanilines with a reasonable range, further highlighting its synthetic utility.
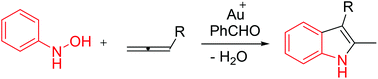

 Please wait while we load your content...
Please wait while we load your content...