New fluorescent bis-dithienylethene (DTE)-based bipyridines as reverse interrupters: single vs. double photochromism†
Abstract
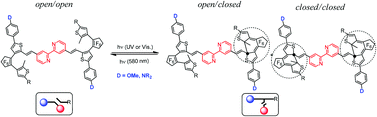
The synthesis and characterization of a series of fluorescent bis-dithienylethene (DTE)-based bipyridines, where the donor (D) and acceptor (A) groups are located on the same thiophene ring of the DTE unit, and their zinc(II) and rhenium(I) complexes are reported. Their photochromic properties have been investigated by UV-visible and 1H NMR spectroscopy. These studies reveal that in non-polar solvents it is possible to modulate the photoreactivity, single vs. double ring-closure, by changing the nature of the donor group. The solvent effect, as well as the influence of the organometallic moieties on the photochromic behavior of these molecules, is also discussed. Finally, upon photoconversion to the photostationary state (PSS), a quenching of fluorescence is observed for the bipyridine ligands, due to disruption of the conjugation upon ring-closing.

 Please wait while we load your content...
Please wait while we load your content...