First example of quinine-squaramide catalyzed enantioselective addition of diphenyl phosphite to ketimines derived from isatins†
Abstract
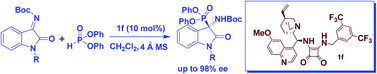
A highly enantioselective addition of diphenyl phosphite to ketimines derived from isatins has been achieved using a bifunctional organocatalyst, quinine-derived squaramide catalyst. This method works efficiently with several ketimines to produce the corresponding 3-amino-2-oxoindolin-3-yl-phosphonates in excellent yields with high enantioselectivity (up to 98% ee).

 Please wait while we load your content...
Please wait while we load your content...