Is the 2,3-carbon–carbon bond of indole really inert to oxidative cleavage by Oxone? – Synthesis of isatoic anhydrides from indoles†
Abstract
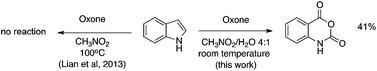
A recent report has indicated that the oxidizing agent Oxone does not possess the ability to cleave the 2,3-carbon–carbon bond of indole. Work in our laboratory shows that this is not the case. Indole and a variety of aryl ring substituted derivatives readily react to form synthetically important isatoic anhydrides.

 Please wait while we load your content...
Please wait while we load your content...