The development of highly active acyclic chiral hydrazides for asymmetric iminium ion organocatalysis†
Abstract
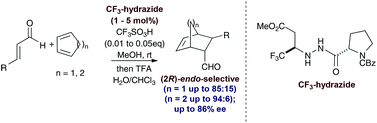
Double asymmetric induction has been employed as a tool to optimise pyrazolidinone-derived organocatalysts for the asymmetric iminium ion catalysed Diels–Alder reaction. Mechanistic studies revealed a superior hydrazide catalyst deriving from methanolysis of the chiral pyrazolidinone precursor. This catalyst displays unusually high endo diastereoselectivity and good enantioselectivity with a range of β-arylenals and cyclic dienes at catalyst loadings as low as 1 mol%.

 Please wait while we load your content...
Please wait while we load your content...