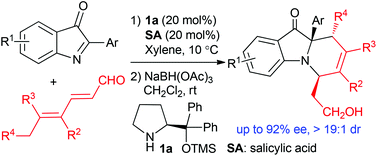
An asymmetric normal-electron-demand aza-Diels–Alder reaction via trienamine catalysis†
Abstract
An asymmetric normal-electron-demand aza-Diels–Alder cycloaddition of 2-aryl-3H-indol-3-ones and 2,4-dienals was explored via trienamine catalysis of a chiral secondary amine. Multifunctional tricyclic polyhydropyrido[1,2-a]indoles were efficiently constructed in good stereoselectivity (up to 92% ee, >19 : 1 dr).

 Please wait while we load your content...
Please wait while we load your content...