In situ approach for testing the enantiopurity of chiral amines and amino alcohols by 1H NMR†
Abstract
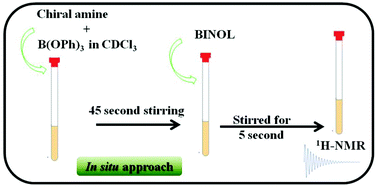
An in situ approach involving a simple mix and shake method for testing the enantiopurity of primary, secondary and tertiary chiral amines and their derivatives, chiral amino alcohols, by 1H-NMR spectroscopy is developed. The protocol involves the in situ formation of chiral ammonium borate salt from a mixture of C2 symmetric chiral BINOL, trialkoxyborane and chiral amines. The proposed concept was demonstrated convincingly on a large number of chiral and pro-chiral amines and amino alcohols, and also aids the precise measurement of enantiomeric excess. The protocol can be completed in a couple of minutes directly in the NMR sample tube, without the need for any physical separation.

 Please wait while we load your content...
Please wait while we load your content...