Enantioselective inhibition of reverse transcriptase (RT) of HIV-1 by non-racemic indole-based trifluoropropanoates developed by asymmetric catalysis using recyclable organocatalysts
Abstract
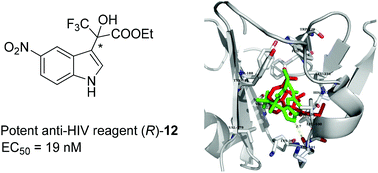
Herein, we report the development of efficient inhibitors of reverse transcriptase (RT) of HIV-1 based on indole-alkyl trifluoropyruvate derivatives by a TZM-bl cell assay. The inhibitory activities of the two enantiomers and the corresponding racemic mixture have been compared. TZM-bl cells exhibited strong enantioselective discrimination for the (R)-configuration, among these indole derivatives, the most active compound R-12, with a 5-NO2 substituent, gave the best result when tested in the TZM-bl cells on HIV virus type HIV-1IIIB, with an EC50 value of 0.019 μM, CC50 value of 210.697 μM and SI (selectivity index, CC50/EC50) value of 11 089, respectively. The cell test showed that, in most cases, the R-enantiomer was superior to the Rac-mixture, which was better than the corresponding S-enantiomer. The results indicated that the R-enantiomer is the most favorable configuration as an efficient HIV-1 inhibitor. Molecular modeling studies suggested a structural basis for the enantioselectivity of RT towards this class of molecules.

 Please wait while we load your content...
Please wait while we load your content...