Fast preparation of an N-acetylglucosaminylated peptide segment for the chemoenzymatic synthesis of a glycoprotein
Abstract
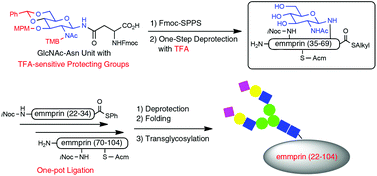
A novel GlcNAc-Asn unit carrying trifluoroacetic acid (TFA)-sensitive O-protecting groups was prepared. The unit was used for the solid-phase peptide synthesis (SPPS) of the N-acetylglucosaminylated emmprin (35–69) thioester via one-step deprotection by TFA combined with the N-alkylcysteine thioesterification method. This segment was used for the synthesis of the first Ig domain (22–104) of emmprin carrying GlcNAc by one-pot ligation with other segments using the thioester method. Finally, the sugar chain was elongated by transglycosylation using glycosynthase to give the Ig domain carrying the disialo- and asialo-complex-type sugar chain.

 Please wait while we load your content...
Please wait while we load your content...