N-Heterocyclic carbene catalyzed regioselective oxo-acyloxylation of alkenes with aromatic aldehydes: a high yield synthesis of α-acyloxy ketones and esters†
Abstract
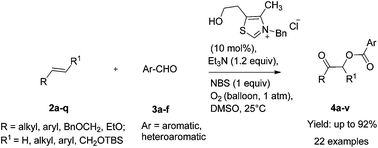
An N-heterocyclic carbene (NHC)-catalyzed reaction of alkenes with aromatic aldehydes providing for a high yield synthesis of α-acyloxy ketones and esters has been described. This unprecedented regioselective oxidative process employs NBS and Et3N in stoichiometric amounts and O2 (1 atm) as an oxidant under ambient conditions in DMSO as a solvent.

 Please wait while we load your content...
Please wait while we load your content...