Chiral olefin–sulfoxide as ligands for rhodium-catalyzed asymmetric conjugate addition of arylboronic acids to unsaturated esters†
Abstract
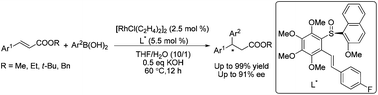
An efficient rhodium/olefin–sulfoxide catalyzed asymmetric conjugate addition of organoboronic acids to various unsaturated esters has been developed, where 2-methoxy-1-naphthyl sulfinyl functionalized olefin ligands have been shown to be highly effective, and are especially applicable to unsaturated methyl esters with up to 99% yield and 91% ee.

 Please wait while we load your content...
Please wait while we load your content...