An efficient chemical synthesis of carboxylate-isostere analogs of daptomycin†
Abstract
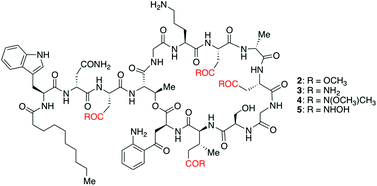
Herein we report a direct and efficient method for the synthesis of four new carboxylate-isostere analogs of
- This article is part of the themed collection: In Celebration of Andrew D. Hamilton’s Career in Chemistry

 Please wait while we load your content...
Please wait while we load your content...