Concise total syntheses of (±)-noruleine and (±)-uleine†
Abstract
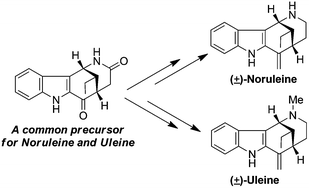
The first total synthesis of (±)-noruleine and a concise synthesis of (±)-uleine have been accomplished via the DDQ mediated dehydrogenative cyclization of a tetrahydrocarbazole derivative bearing a non-substituted amide functionality to prepare the key azocino[4,3-b]indole precursor.

 Please wait while we load your content...
Please wait while we load your content...