Substrate scope and synthetic applications of the enantioselective reduction of α-alkyl-β-arylenones mediated by Old Yellow Enzymes†
Abstract
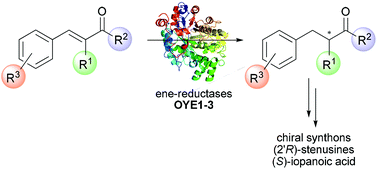
The ene-reductases mediated bioreduction of a selection of open-chain α-alkyl-β-aryl enones afforded the corresponding saturated α-chiral ketones in high yield and optical purity in several cases. The stereo-electronic requirements of the reaction have been investigated, considering the nature and location of substituents on the aromatic ring as well as the steric hindrance at the α-position and adjacent to the carbonyl functionality. The general considerations drawn allow us to guide the design of α,β-unsaturated ketones to be employed as substrates of ene-reductases in future preparative applications. An interesting case of orthogonality between enzyme-based and substrate-based stereocontrol within the highly homologous ene-reductases from Saccharomyces species (OYE1–3) has been reported and rationalized with the help of computational docking studies. Furthermore, to demonstrate the synthetic versatility of the reaction, the key chiral precursors of biologically active compounds such as (2′R)-stenusines and (S)-iopanoic acid were obtained. The very robust protocol allowed us to run the reactions on preparative scale in quantitative yields, with a simple work-up and no chromatographic purification steps.

 Please wait while we load your content...
Please wait while we load your content...