Synergistic effects within a C2-symmetric organocatalyst: the potential formation of a chiral catalytic pocket†
Abstract
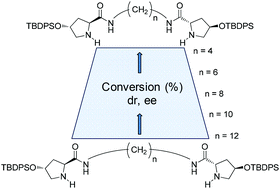
This study describes the synthesis of five novel C2-symmetric organocatalysts that facilitate the on-water asymmetric
* Corresponding authors
a
Strategic Research Centre, Faculty of Science and Technology, Deakin University, Geelong, Victoria, Australia
E-mail:
luke.henderson@deakin.edu.au
Fax: (+613) 52271045
Tel: (+613)52272767
b Institute for Frontier Materials, Deakin University, Geelong, Victoria, Australia
This study describes the synthesis of five novel C2-symmetric organocatalysts that facilitate the on-water asymmetric

 Please wait while we load your content...
Something went wrong. Try again?
Please wait while we load your content...
Something went wrong. Try again?
J. P. Delaney, H. L. Brozinski and L. C. Henderson, Org. Biomol. Chem., 2013, 11, 2951 DOI: 10.1039/C3OB27250H
This article is licensed under a Creative Commons Attribution 3.0 Unported Licence. You can use material from this article in other publications without requesting further permissions from the RSC, provided that the correct acknowledgement is given.
Read more about how to correctly acknowledge RSC content.
 Fetching data from CrossRef.
Fetching data from CrossRef.
This may take some time to load.
Loading related content
